Molar mass of CO_2=44g/mol
No of moles=1.5mol
Mass of CO_2

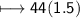
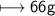
And
Molar mass of water=18g/mol
No of moles=2
Mass of H_2O
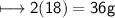
Total mass of products
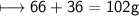
EMPIRICAL FORMULA:-CH_2O_3
Empirical formula mass:-
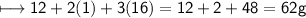
We need n now
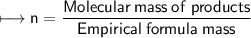
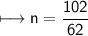
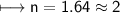
Now
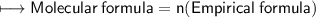
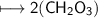
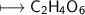
It's Ethene Oxide .
Answer is Ethene(C_2H_4)
Option B is correct
Done