As you can probably guess, the term "reactants" are the chemicals or elements that react to one another. They mix and combine. The reactants are the start of the process while the products are the final result or the end of the process. With the "process" being the chemical reaction overall.
To help remember that product comes after reactant, you can think of "final product".
So reactants combine to form products.
The order can be written like this
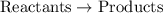
For example, combining the hydrogen (H) and oxygen (O) will get us
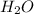 which is water.
which is water.
More technically, we'd write
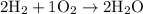
The reactants here are the
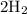 (two copies of double hydrogen atoms) and
(two copies of double hydrogen atoms) and
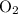 (one copy of the double oxygen atoms).
(one copy of the double oxygen atoms).
The product in this example is
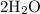 (two copies of water molecules)
(two copies of water molecules)
--------------------------------------
As another example,
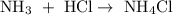
The two reactants here are
and those reactants combine to form the product of
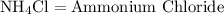
----------------------------------------
It's possible to have more than one product as this formula shows
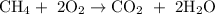
Reactants are:
Products are:
In some diagrams, you'll see heat/energy being included on either the reactant side or the product side. If you want to focus on just the chemical formula itself, then you can leave it out.
That formula shown at the top of this section effectively is saying "if you burn methane, then you'll get carbon dioxide and water". The process of burning usually involves oxygen.