Answer:
Approximately
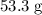 .
.
Step-by-step explanation:
Lookup Avogadro's Number:
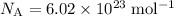 (three significant figures.)
(three significant figures.)
Lookup the relative atomic mass of
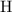 ,
,
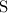 , and
, and
 on a modern periodic table:
on a modern periodic table:
(For example, the relative atomic mass of
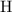 is
is
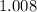 means that the mass of one mole of
means that the mass of one mole of
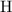 atoms would be approximately
atoms would be approximately
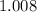 grams on average.)
grams on average.)
The question counted the number of
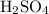 molecules without using any unit. Avogadro's Number
molecules without using any unit. Avogadro's Number
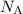 helps convert the unit of that count to moles.
helps convert the unit of that count to moles.
Each mole of
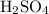 molecules includes exactly
molecules includes exactly
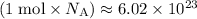 of these
of these
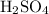 molecules.
molecules.
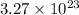
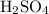 molecules would correspond to
molecules would correspond to
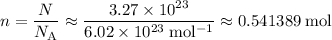 of such molecules.
of such molecules.
(Keep more significant figures than required during intermediary steps.)
The formula mass of
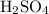 gives the mass of each mole of
gives the mass of each mole of
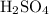 molecules. The value of the formula mass could be calculated using the relative atomic mass of each element:
molecules. The value of the formula mass could be calculated using the relative atomic mass of each element:
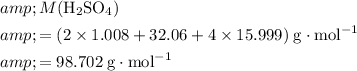 .
.
Calculate the mass of approximately
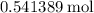 of
of
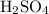 :
:
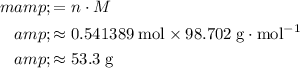 .
.
(Rounded to three significant figures.)