Answer:
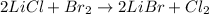
Single displacement reaction.
Step-by-step explanation:
Hello!
In this case, by firstly rearranging the chemical reaction as shown below:
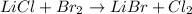
It is also necessary to balance it at follows:
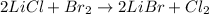
Thus, since bromine and chlorine are being exchanged as a result of the atoms rearrangement, we infer this is a single displacement reaction.
Best regards!