Answer:
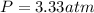
Step-by-step explanation:
Hello!
In this case, since know the volume, temperature and pressure of the initial containers, we can compute the moles of each gas prior to the opening of the valve as shown below:
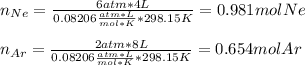
Next, we add them up to obtain the total moles:
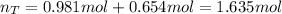
Now, the total volume:
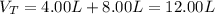
Finally, the total pressure is computed by using the ideal gas equation:
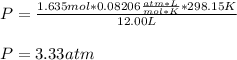
Best regards!