Answer:
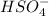 is the Bronsted Lowry acid.
is the Bronsted Lowry acid.
Step-by-step explanation:
Hello there!
In this case, since the reaction:
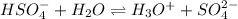
Shows that the hydrogen sulfate ion provides one hydrogen ion to water to form H₃O⁺, we can infer that the former is the Bronsted Lowry acid and the latter is the Bronsted Lowry base as it receives the given away hydrogen ions.
Best regards!