Answer: Yes, magnesium will replace hydrogen because magnesium is higher on the activity series list.
Step-by-step explanation:
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution.
A general single displacement reaction can be represented as :
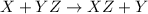
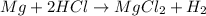
When magnesium is added to hydrochloric acid, magnesium being more reactive than hydrogen displaces hydrogen atom its salt solution and lead to formation of magnesium chloride and hydrogen gas.