Answer:
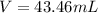
Step-by-step explanation:
Hello!
In this case, since the reaction between sulfuric acid and aluminum hydroxide is:
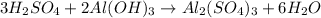
Whereas the ratio of sulfuric acid to aluminum hydroxide is 3:2; thus, we first compute the moles of sulfuric acid that complete react with 3.209 g of aluminum hydroxide:
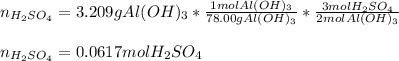
Then, given the molarity, it is possible to obtain the milliliters as follows:
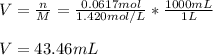
Best regards!