Answer:
D.
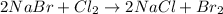
Step-by-step explanation:
Hello!
In this case, for the given set of chemical reactions, it is possible to infer that D. is a categorized as redox due to the following:
Since both chlorine and bromine remain as diatomic gases, their oxidation states in such a form is 0, but as anions with lithium cations they have a charge of - according to the following reaction and half-reactions:
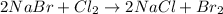
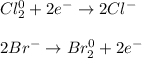
Unlike the other reactions whereas no change in the oxidation states is evidenced.