Answer:
Step-by-step explanation:
Hello!
In this case, when methane is burnt in the presence of carbon dioxide, the following chemical reaction is carried out:
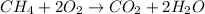
Thus, since there is a 1:2 mole ratio between methane and oxygen, we set up the following mathematical expression to obtain the produced mass of carbon dioxide as a result of the reaction:
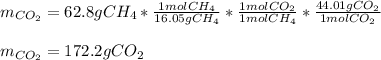
Best regards!