Answer:
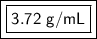
Step-by-step explanation:
We are asked to find the density of a small object. Density is calculated using the following formula.
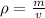
The mass of the object is 68.1 grams.
The volume was found using water displacement. A known quantity of water was measured out (25.0 mL), then the object was added. The new water level was recorded (43.3 mL). The volume is the difference between the two water levels.
- volume = final water level - initial water level
- volume = 43.3 mL - 25 mL = 18.3 mL
Now we know the mass and the volume, so we can substitute the values into the formula.
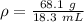
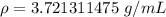
The original measurements of mass and water levels have 3 significant figures, so our answer must have the same. For the number we found, that is the hundredth place. The 1 in the thousandth place tells us to leave the 2 in the hundredth place.
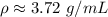
The density of the small object is approximately 3.72 grams per milliliter.