Answer:
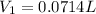
Step-by-step explanation:
Hello there!
In this case, since we need to dilute the 1.75-M HCl, and the total number of moles remain unchanged, we can write:
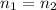
And in terms of volume and concentration:
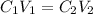
Thus, we can solve for the volume of the concentrated HCl as shown below:
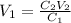
Therefore, we plug in the data to get:
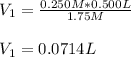
Best regards!