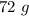Answer:

Step-by-step explanation:
To convert from moles to grams, we must use the molar mass (also known as the gram formula mass).
First, look up the molar masses of the elements in the formula.
- C: 12.011 g/mol
- H: 1.008 g/mol
- O: 15.999 g/mol
Next, multiply by the subscript, because it tells us the number of atoms of each element in the formula.
- C₉: 4(12.011 g/mol)= 108.099 g/mol
- H₈: 8(1.008 g/mol)= 8.064 g/mol
- O₄: 4(15.999 g/mol)= 63.996 g/mol
Add the values.
- 108.099 + 8.064+63.996=180.159 g/mol
Use this molar mass as a ratio.
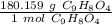
Multiply by the given number of moles, 0.40
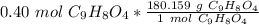
The units moles of aspirin cancel.
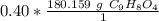
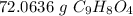
The original number of moles has 2 sig figs (4 and 0), so answer must have the same. For the number we calculated, it is the ones place. The 0 in the tenth place tells us to leave the 2.