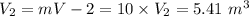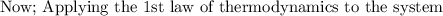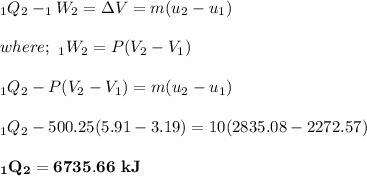Answer:
Step-by-step explanation:
From the given information:
At state 1:
Initial Quality
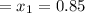
mass = 10.0 kg
At state 2:
Temperature
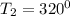
mass of the piston
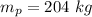
area of the piston
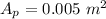
Atmospheric pressure
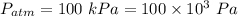
Gravitational acceleration = 9.81 m/s²
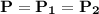 , This is because there exists no restriction to the movement of the piston and provided the process is frictionless. So, the process 1-2 is regarded as constant.
, This is because there exists no restriction to the movement of the piston and provided the process is frictionless. So, the process 1-2 is regarded as constant.
To calculate the applying force balance over the piston by using force balance in the vertical direction:
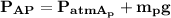
∴
(100 × 10³)×0.005 + 204 × 9.31 = P × 0.05
P = 500248 Pa
P = 500.25 kPa
At state 1:
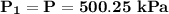
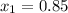
Hence, this is a saturated mixture of liquid and vapor
Using the steam tables at 500.25 kPa
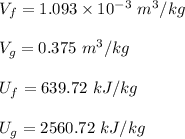
∴
Specific volume at state 1 is given as:
![V_1 = [ V_f +x_1(v_g -v_f) ] \ at \ 500.25 \ kPa \\ \\ V_1 = 0.319 \ m^3/kg](https://img.qammunity.org/2022/formulas/physics/college/8ax389smiucebio28mttv9aeasifibnixf.png)
volume at state 1 is given by:
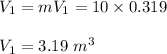
Similarly, the specific internal energy is:
![U_1 = [U_f +x_1 (U_o-Uf)] \ at \ 500.25 \ kPa](https://img.qammunity.org/2022/formulas/physics/college/adyi8azob9lwwbgnbb37jvb5y19hijb32w.png)
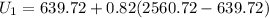
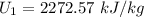
At state 2:
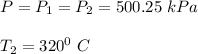
Using steam tables at P = 500.25 kPa and T = 320° C
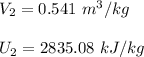
∴