Solution :
Partition coefficient Kd

= 9.0
A). 1 x 200 mL extraction :
Let m be the mass of caffeine in the water
Mass of caffeine in
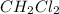 = 100 - m
= 100 - m
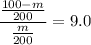
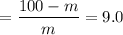
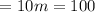
m = 10
Mass remaining in coffee = m = 10 mg
B). 2 x 100 mL extraction :
First extraction :
Let
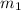 be the mass of caffeine in the water
be the mass of caffeine in the water
Mass of caffeine in
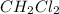 =
=
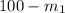
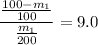
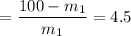
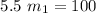
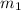 = 18.18
= 18.18
Mass remaining in coffee =
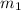 = 18.18 mg
= 18.18 mg
Second Extraction :
Let
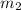 be the mass of caffeine in the water
be the mass of caffeine in the water
Mass of caffeine in
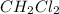 =
=
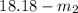
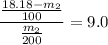
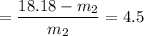
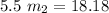
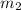 = 3.3
= 3.3
Mass remaining in coffee =
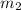 = 3.3 mg
= 3.3 mg