Answer:
Step-by-step explanation:
The formula for determining the energy of state
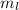 can be computed by using the formula:
can be computed by using the formula:
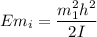
Also, the momentum is:
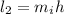
There are 22 electrons with two electrons in each of the lowest II energy levels so that the highest occupied states are
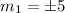
The moment of inertia of an electron on a ring of radius 440 ppm is:
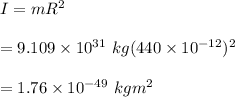
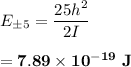
The angular momentum is:
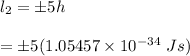
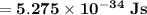
B) Let's recall that:
The lowest occupied energy level is
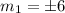 which implies that the energy
which implies that the energy
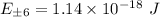
Thus;
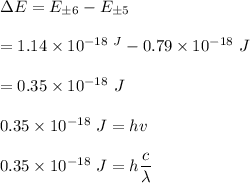
Hence, the radiation which would induce a transition that relates to the wavelength of about 570nm, a wavelength of visible light.