Answer:
Step-by-step explanation:
Lithium is in group 1 so there's 1 outermost shell electron.
Oxygen is in group 6 so there's 6 outermost shell electron.
To react lithium with oxygen, we need 2 lithium and 1 oxygen
Each lithium transfer 1 electron to the oxygen and oxygen gain 2 electrons.
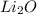 formed.
formed.
If there's 4 lithium oxide, 4 molecules will be there.
If you mean how many ATOMS are there, there will be 3×4=12 atoms.