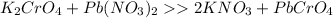Answer:
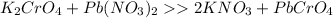
Reactions:
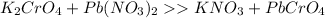
Step-by-step explanation:
In the reaction, the CrO4(Chromate) is a compound NO3(Nitrate) is a compound as well.
Ignore the number in front of the reaction first, calculate how many K, Cr, O, Pb and N in the equation for both left and right side.
Left side: K=2, Cr=1, Pb=1, N=2, O=10
Right side: K=1, Cr=1, Pb=1, N=1, O=7
So we need 3 more oxygen, 1 more nitrogen and 1 more potassium on the right.
If we add a 2 in front of KNO3, there will be 2 potassium, 2 Nitrogen and 6 oxygen, which the reaction complete.
Conclusion: