Answer:
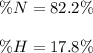
Step-by-step explanation:
Hello there!
In this case, since ammonia is NH₃, we can see nitrogen weights 14.01 g/mol and hydrogen 3.03 g/mol as there is one only nitrogen atom and three hydrogen atoms; thus, the total mass is 17.04 g/mol. In such a way, the percent composition of each element turns out to be:
Best regards!