Answer:
2)
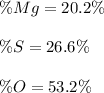
3)
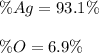
Step-by-step explanation:
Hello!
2) In this case, since magnesium sulfate is MgSO₄, we can see how magnesium weights 24.305 g/mol, sulfur 32.06 g/mol and oxygen 64.00 g/mol as there is one atom of magnesium as well as sulfur but four oxygen atoms for a total of g/mol; thus the percent compositions are:
3) In this case, although the element seems to contain Ag and O, we infer its molecular formula is Ag₂O; thus, since we have two silver atoms weighing 215.74 g/mol and one oxygen atom weighing 16.00 g/mol for a total of 231.74 g/mol, we obtain the following percent compositions:
Best regards!