Answer:
V = 5.97 L
Step-by-step explanation:
Hello!
In this case, according to the ideal gas law:

We would need to solve for V as we know the temperature and pressure as the gas it at STP conditions (273 K and 1 atm respectively):
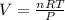
Next, we compute the moles in 8 g of NO, given its molar mass of 30.01 g/mol:
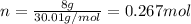
Therefore, we obtain the following volume:

Best regards!