Answer:
pH = 5.54
Step-by-step explanation:
The pH of a buffer solution is given by the Henderson-Hasselbach (H-H) equation:
- pH = pKa + log
![([CH_3COO^-])/([CH_3COOH])](https://img.qammunity.org/2022/formulas/chemistry/college/wumiv2xydcjvvhznac10qtc3xz82zf5y5j.png)
For acetic acid, pKa = 4.75.
We calculate the original number of moles for acetic acid and acetate, using the given concentrations and volume:
- CH₃COO⁻ ⇒ 0.377 M * 0.250 L = 0.0942 mol CH₃COO⁻
- CH₃COOH ⇒ 0.345 M * 0.250 L = 0.0862 mol CH₃COOH
The number of CH₃COO⁻ moles will increase with the added moles of KOH while the number of CH₃COOH moles will decrease by the same amount.
Now we use the H-H equation to calculate the new pH, by using the new concentrations:
- pH = 4.75 + log
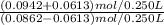 = 5.54
= 5.54