Answer: trigonal bipyramidal
Step-by-step explanation:
Number of electron pairs =
![(1)/(2)[V+N-C+A]](https://img.qammunity.org/2022/formulas/chemistry/college/l1uglqqs0kibv7azdl2u21sghqc7blybqj.png)
V = number of valence electrons present in central atom
N = number of monovalent atoms bonded to central atom
C = charge of cation
A = charge of anion
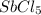 :
:
In the given molecule, antimony is the central atom and there are five chlorine as monovalent atoms.
The number of electron pairs are 5 that means the hybridization will be
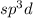 and geometry of the molecule will be trigonal bipyramidal.
and geometry of the molecule will be trigonal bipyramidal.