Answer:
17.3 g
Step-by-step explanation:
Given the following data;
- Quantity of heat, Q = 0.507 J
- Temperature = 0.007°C
- Specific heat capacity of water = 4.2 J/g°C
Mathematically, Heat capacity is given by the formula;
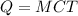
Where;
- Q represents the heat capacity or quantity of heat.
- M represents the mass of an object.
- C represents the specific heat capacity of water.
- T represents the temperature.
Making "M" the subject of formula, we have;
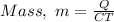
Substituting the values into the formula, we have;
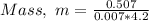
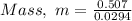
Mass, m = 17.3 grams