Answer:
Initial temperature, T1 = 99.4 Kelvin
Step-by-step explanation:
Given the following data;
- Initial volume, V1 = 65.8 Litres
- Final temperature, T2 = 200 Kelvin
- Final volume, V2 = 132.4 Litres
To find the initial temperature (T1), we would use Charles' law;
Charles states that when the pressure of an ideal gas is kept constant, the volume of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Charles' law is given by the formula;
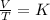
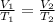
Making T1 as the subject formula, we have;
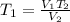
Substituting the values into the formula, we have;
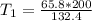
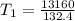
Initial temperature, T1 = 99.4 Kelvin