Answer:
The final volume of the balloon is 24.480 liters.
Step-by-step explanation:
Let consider that no leakages occur in the balloon during the isobaric process and that helium behaves ideally. From Equation of State for Ideal Gases, we construct the following relationship:
 (1)
(1)
Where:
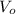 ,
,
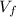 - Initial and final volume, measured in liters.
- Initial and final volume, measured in liters.
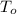 ,
,
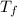 - Initial and final temperature, measured in Kelvin.
- Initial and final temperature, measured in Kelvin.
If we know that
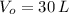 ,
,
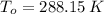 and
and
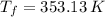 , the final volume of the balloon is:
, the final volume of the balloon is:

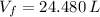
The final volume of the balloon is 24.480 liters.