Answer: The given amount of
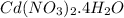 contains 0.430 moles of Cd, 0.860 moles of N and
contains 0.430 moles of Cd, 0.860 moles of N and
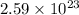 molecules of water.
molecules of water.
Step-by-step explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
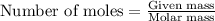 ......(1)
......(1)
Given mass of
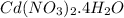 = 132.4 g
= 132.4 g
Molar mass of
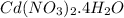 = 308.5 g/mol
= 308.5 g/mol
Plugging values in equation 1:
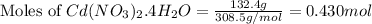
1 mole of
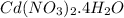 contains 1 mole of Cd, 2 moles of nitrogen atom (N), 10 moles of oxygen atom (O) and 8 moles of hydrogen atom (H).
contains 1 mole of Cd, 2 moles of nitrogen atom (N), 10 moles of oxygen atom (O) and 8 moles of hydrogen atom (H).
So, 0.430 moles of
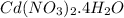 will contain =
will contain =
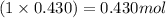 of Cd and
of Cd and
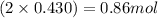 of N
of N
According to the mole concept:
1 mole of a compound contains
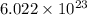 number of molecules
number of molecules
So, 0.430 moles of
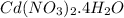 will contain =
will contain =
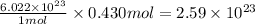 number of water molecules.
number of water molecules.
Hence, the given amount of
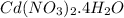 contains 0.430 moles of Cd, 0.860 moles of N and
contains 0.430 moles of Cd, 0.860 moles of N and
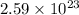 molecules of water.
molecules of water.