Answer:

Step-by-step explanation:
We are asked to find the volume of ammonia gas given a change in pressure. We will use Boyle's Law, which states the volume of a gas is inversely proportional to the pressure of a gas. The formula is:
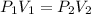
The ammonia gas originally occupies a volume of 450 milliliters at a pressure of 720 millimeters of mercury. Substitute the values into the formula.
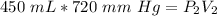
The pressure is changed to standard atmospheric pressure, which is 760 millimeters of mercury. The new volume is unknown.
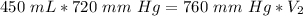
We are solving for the volume at standard pressure. We will need to isolate the variable V₂. It is being multiplied by 760 millimeters of mercury. The inverse of multiplication is division. Divide both sides of the equation by 760 mm Hg.
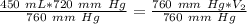
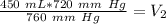
The units of millimeters of mercury (mm Hg) cancel.
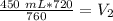
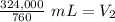
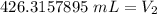
The original values of volume and pressure have 3 significant figures. Our answer must have the same. For the number we calculated, that is the ones place. The 3 in the tenths place tells us to leave the 6 in the ones place.
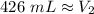
The volume at standard atmospheric pressure is approximately 426 milliliters.