Answer:
Yes, pure NaCl will precipitate out of the solution
Step-by-step explanation:
The parameters of the NaCl solution and HCl mixture are;
The saturation of the NaCl solution = Saturated solution
The mass concentration of the NaCl solution = 317 g/L
The volume of the HCl added = 10 mL
The concentration of the HCl added = 13.0 M HCl
The volume of the saturated NaCl solution = 0.2 L
The molar mass of NaCl = 58.44 g/mol
The number of moles of NaCl per liter = (317 g/L)/(58.44 g/mol) = 7925/1461 moles ≈ 5.42 moles
The ionization of the molecules in solution are;
HCl (aq) + H₂O (l) → H₃O⁺ (aq) + Cl⁻ (aq)
NaCl (s) ↔ Na⁺ (aq) + Cl⁻ (aq)
The number of moles of the Cl⁻ ion from the HCl is 13 moles per liter, the number of moles of Cl⁻ ion from the saturated solution of NaCl is approximately 5.42 moles, therefore, ionic product I.P. of the solution of Cl⁻ ions is larger than the solubility product of the solution,
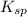 of the NaCl and according to Le C h a t e l i e r's principle, NaCl will precipitate out of the solution
of the NaCl and according to Le C h a t e l i e r's principle, NaCl will precipitate out of the solution