Answer:
The water vapor will have the greatest entropy at the triple point
Step-by-step explanation:
At the triple point of water, which has the properties of 0.0075°C temperature and 6.11657 mbar pressure, solid ice, liquid water and water vapor exist together in a location
According to Professor Stephen Lower on LibreTexts website entropy is a measure the extent to which thermal energy is shared and spread in a system
The change in entropy, ΔS = ΔH/T
The heat change in
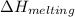 = 6.01 kJ/mol
= 6.01 kJ/mol
The heat change in vaporization,
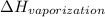 = 45.05 kJ/mol
= 45.05 kJ/mol
The heat change in sublimation,
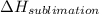 = 51.06 kJ/mol
= 51.06 kJ/mol
Therefore, the entropy of the water vapor
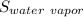 > The entropy of liquid water,
> The entropy of liquid water,
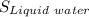 > The entropy of the solid ice,
> The entropy of the solid ice,
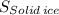
The portion with the highest entropy at the triple point is the water vapor
The water vapor will have the greatest entropy at the triple point