Answer:
d. 7.7x10^23 molecules
Step-by-step explanation:
Given the following data:
Mass of water (H2O) = 23g
To find the number of molecules;
First of all, we would determine the number of moles;
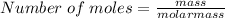
Molar mass of water (H2O) = (1 * 2) + 16 = 18 g/mol
Substituting into the equation, we have
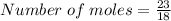
Number of moles = 1.2778 moles
Now, to find the number of water molecules;
We know that Avogadro constant is equal to 6.02 * 10^23 mol¯¹
Number of water molecules = number of H2O moles * Avogadro constant
Substituting into the equation, we have;
Number of water molecules = 1.2778 × 6.02 * 10^23
Number of water molecules = 7.7 × 10^23 atoms.