Step-by-step explanation:
The mass of a pot is 300g and contains 90% aluminum. Find the number of moles of aluminum in the pot. P.A. (Al = 27)
The mass of aluminum present in the pot is:
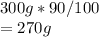
Hence, in the given pot 270g Al is present.
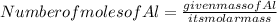
The gram atomic mass of Al -27 g/mol
Given the mass of Al is 270 g
Substitute these values in the above formula:
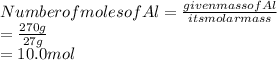
Answer is 10.0 mol of Al is present.