Answer: The formic acid is 3.5 times stronger than acetic acid.
Step-by-step explanation:
The strength of an acid depends on its acid dissociation constant.
For a hypothetical monoprotic acid HA, the dissociation equilibrium is:
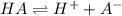
![K_a=([H^+][A^-])/([HA])](https://img.qammunity.org/2022/formulas/chemistry/college/2vtq1vo876w1v4cce6cdcyzxss8v1p1ryp.png)
More is the value of
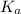 , greater is the dissociation and stronger is the acid.
, greater is the dissociation and stronger is the acid.
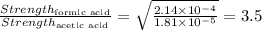
Thus formic acid is 3.5 times stronger than acetic acid.