Answer:

Step-by-step explanation:
We are asked to find how many moles of gas occupy a volume of 101.3 liters.
1 mole of any gas at STP (standard temperature and pressure) has a volume of 22.4 liters. We can use this information to make a proportion.
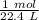
We are converting 101.3 liters to moles, so we multiply the proportion by that value.
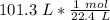
The units of liters (L) cancel.
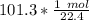
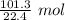
Divide.
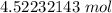
The original value of liters (101.3 L) has 4 significant figures, so our answer must have the same. For the number we calculated, that is the thousandths place. The 3 in the ten-thousandths place to the right tells us to leave the 2 in the thousandths place.
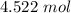
101.3 liters of gas is equal to approximately 4.522 moles of gas.