Answer:
Part 1: Sodium and Rubidium.
Part 2:
Protons = 12
reason: protons are equal to the atomic number of an atom. For Magnesium, atomic number is 12.
Neutrons:
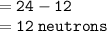
reason: neutrons is given by formula:

where mass number of magnesium is 24.
electrons: = 12 electrons
reason: Magnesium atom has electrons summing up to 12