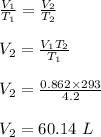Answer:
(a) The volume of the liquid helium at 25 K is 5.13 L
(b) The volume of the liquid helium at 293 K is 60.14 L.
Step-by-step explanation:
Given;
mass of the liquid helium, m = 10 g
initial temperature of the liquid helium, T₁ = 4.2 K
pressure of the liquid helium, P = 1.00 atm
Atomic mass of Helium, = 4 g
number of moles of Helium, n = 10 / 4 = 2.5 moles
The initial volume of the liquid helium is calculated as;
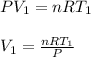
where;
R is ideal gas constant, = 0.08205 L.atm./mol.K
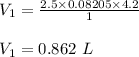
(a) The volume of the liquid helium at 25 K.
Apply Charles law;
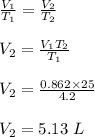
(b) The volume of the liquid helium at 293 K.