Answer:
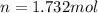
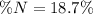
Step-by-step explanation:
Hello!
In this case, since the molecular formula of glycine is C₂H₅NO₂, we realize that the molar mass is 75.07 g/mol; thus, the moles in 130.0 g of glycine are:
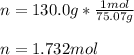
Furthermore, we can notice 75.07 grams of glycine contains 14.01 grams of nitrogen; thus, the percent nitrogen turns out:
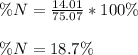
Best regards!