Answer: The percent dissociation of butanoic acid is 9.8%
Step-by-step explanation:
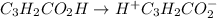
cM 0 0

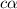
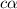
So dissociation constant will be:
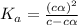
Give c= 1.4 mM =
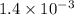 and
and
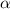 = dissociation constant
= dissociation constant
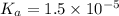
Putting in the values we get:
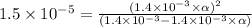
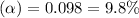
Thus percent dissociation of butanoic acid is 9.8%