Answer:
The mass of oxygen the container must have is 150.85 g.
Step-by-step explanation:
Given;
mass of the oxygen, m₁ = 44.5 g
initial pressure of the gas, P₁ = 2.3 atm
final pressure of the gas, P₂ = 7.8 atm
Atomic mass of oxygen gas, = O₂ = 16 x 2 = 32 g
initial number of moles of oxygen in the container, n₁ = 44.5/32 = 1.39
let the final number of moles of oxygen = n₂
Apply ideal gas equation;
PV = nRT
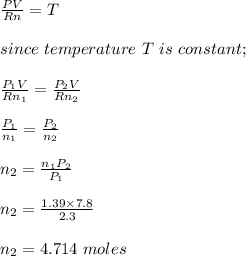
The mass of the oxygen in grams is calculated as;
m₂ = 4.714 x 32g
m₂ = 150.85 g
Therefore, the mass of oxygen the container must have is 150.85 g.