Answer:
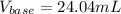
Step-by-step explanation:
Hello!
In this case, according to the chemical reaction by which HBr reacts with Ba(OH)2:
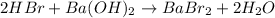
We can see there is a 2:1 mole ratio between the acid and the base; thus, at the equivalent point we can write:
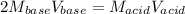
Therefore, for is to compute the volume of the used base, we proceed as shown below:
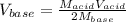
And we plug in to obtain:

Best regards!