Answer:
1) 2.69 * 10²³ PBr₅
2) 6.02 * 10²⁴ C₁₂H₂₂O₁₁
Step-by-step explanation:
Question 1)
We want to convert 192 grams of phosphorus pentabromide to molecules. Note that 192 is three significant figures.
Phosphorus pentabromide is given by PBr₅.
To convert from grams to molecules, we can convert from grams to moles first, and then from moles to molecules.
To convert from grams to moles, we will find the molar mass of PBr₅.
Since the molar mass of P is 30.974 g/mol and the molar mass of Br is 79.904 g/mol, the molar mass of PBr₅ is:
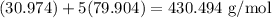
And since we want to convert from grams to moles, we can write the following ratio:
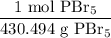
Where grams is in the denominator, which allows us to cancel them out, leaving us with only moles.
To convert from moles to molecules, we can use the definition of the mole: a mole of one substance has 6.022 * 10²³ amount of that substance.
So, a mole of PBr₅ has 6.022 * 10²³ molecules of PBr₅. Since we want to cancel out the moles, we can write the ratio:
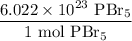
In combination, starting with 192 grams of PBr₅, we will acquire:
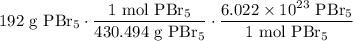
Cancel like units:
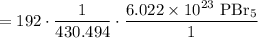
Multiply. Hence:
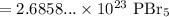
Since the final answer should have three significant digits, our final answer is:
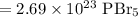
So, there are about 2.69 * 10²³ molecules of PBr₅ in 192 grams of the substance.
Question 2)
We want to convert 3.42 kilograms of table sugar (C₁₂H₂₂O₁₁) to molecules. Note that this is three significant figures.
3.42 kilograms is equivalent to 3420 grams of table sugar.
Again, we can convert from grams to moles, and then from moles to molecules.
First, we will find the molar mass of table sugar. The molar mass of carbon is 12.011 g/mol, hydrogen 1.008 g/mol, and oxygen 15.999 g/mol. Thus, the molar mass of table sugar will be:
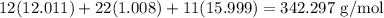
To cancel units, we can write our ratio as:
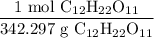
With grams in the denominator.
And by definition:
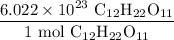
Combining the two ratios and the starting value, we acquire:
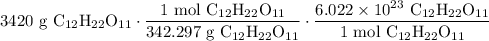
Cancel like units:
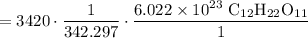
Multiply:
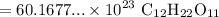
Rewrite:
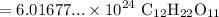
The resulting answer should have three significant digits. Hence:
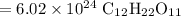
So, there are about 6.02 * 10²⁴ molecules of table sugar in 3.42 kilograms of the substance.