Step-by-step explanation:
Given the mass of HCl is ---- 0.50 g
The volume of solution is --- 4.0 L
To determine the pH of the resulting solution, follow the below-shown procedure:
1. Calculate the number of moles of HCl given by using the formula:
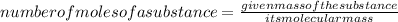
2. Calculate the molarity of HCl.
3. Calculate pH of the solution using the formula:
![pH=-log[H^+]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/rhi7zd8f68j2z6w78gdggu.png)
Since HCl is a strong acid, it undergoes complete ionization when dissolved in water.
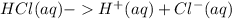
Thus,
![[HCl]=[H^+]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/dbbwgbl982554w6ixsqhiq.png)
Calculation:
1. Number of moles of HCl given:
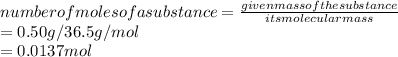
2. Concentration of HCl:
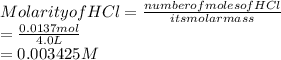
3. pH of the solution:
![pH=-log[H^+]\\=-log(0.003425)\\=2.47](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/hyqqwg8qwvn0rru4ds51zw.png)
Hence, pH of the given solution is 2.47.