Answer:
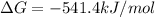
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary to write out the described chemical reaction as shown below:
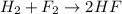
Now, we set up the expression for the calculation of the standard free energy change, considering the free energy of formation of each species, specially those of H2 and F2 which are both 0 because they are pure elements:
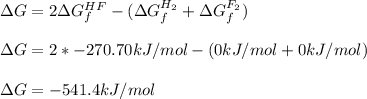
Regards!