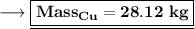Answer:
28.12 kg
Step-by-step explanation:
Here we are given that the density of copper is 8.92 g/ cm³ .
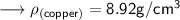
And we are interested in finding out the mass of the given block . The dimensions of the block is 0.052m × 311mm × 19.5 cm .
- Firstly find out the volume in cm³ as the density is in cm³ .
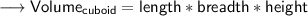
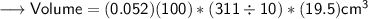
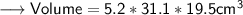
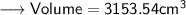
Now we may use Unitary Method to find out the mass of the block as ,
Mass of 1cm³ of copper is 8.92 g .
Mass of 3153.54 cm³ of copper will be 8.92*3153.54 g = 28129.57 g
- And we know that 1kg = 1000g , so