Answer: D. All of the above describe a mole
Step-by-step explanation:
Mole is the unit for amount of substance. It is used for describing large number of particles.
According to avogadro's law, 1 mole of every substance weighs equal to molecular mass and contains avogadro's number
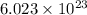 of particles.
of particles.
For example: 1 mole of Na atom will weigh 23 grams and contains
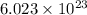 atoms.
atoms.
Thus all the above describes a mole.