Answer: Thus the correct option is D. All of the above describe a mole
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance weighs equal to molecular mass in grams.
1 mole of every substance contains avogadro's number
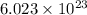 of particles.
of particles.
Mole is the S.I unit for measuring the amount of substance. It is often used to measure large number of particles.
Thus the correct option is D. All of the above describe a mole