Answer:
Substance A will release more heat.
Step-by-step explanation:
Let suppose that both substances experiment an entirely sensible heat process and are incompressible and begin at the same temperature. Physically speaking, specific heat (
 ), measured in kilojoules per kilogram-degree Celsius, can be described by following expression:
), measured in kilojoules per kilogram-degree Celsius, can be described by following expression:
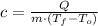 (1)
(1)
Where:
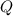 - Released heat, measured in kilojoules.
- Released heat, measured in kilojoules.
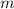 - Sample mass, measured in kilograms.
- Sample mass, measured in kilograms.
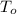 ,
,
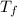 - Initial and final temperatures of the sample, measured in degrees Celsius.
- Initial and final temperatures of the sample, measured in degrees Celsius.
If we know that
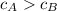 ,
,
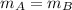 ,
,
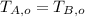 and
and
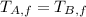 , then we have the following inequation:
, then we have the following inequation:
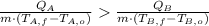
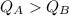
Substance A will release more heat.