Step-by-step explanation:
The volume of given lead nitrate solution is:
52.5 mL.
The amount of lead iodide formed is ---0.248 g.
To get the molarity of lead (II) ion follow the below-shown procedure:
The number of moles of lead iodide formed is:
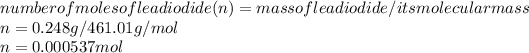
0.000537 mole of lead iodide contains --- 0.000537 moles of lead (II) ion.
Thus, the number of moles are there, volume is there, and to get the molarity of lead (II) ion use the formula:
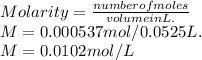
Molarity of lead iodide is --- 0.0102 M.