Answer:
a) 0.684
b) 0.90
Step-by-step explanation:
Catalyst
EO + W → EG
a) calculate the conversion exiting the first reactor
CAo = 16.1 / 2 mol/dm^3
Given that there are two stream one contains 16.1 mol/dm^3 while the other contains 0.9 wt% catalyst
Vo = 7.24 dm^3/s
Vm = 800 gal = 3028 dm^3
hence Im = Vin/ Vo = (3028 dm^3) / (7.24dm^3/s) = 418.232 secs = 6.97 mins
next determine the value of conversion exiting the reactor ( Xai ) using the relation below
KIm =
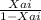 ------ ( 1 )
------ ( 1 )
make Xai subject of the relation
Xai = KIm / 1 + KIm --- ( 2 )
where : K = 0.311 , Im = 6.97 ( input values into equation 2 )
Xai = 0.684
B) calculate the conversion exiting the second reactor
CA1 = CA0 ( 1 - Xai )
therefore CA1 = 2.5438 mol/dm^3
Vo = 7.24 dm^3/s
To determine the value of the conversion exiting the second reactor ( Xa2 ) we will use the relation below
XA2 = ( Xai + Im K ) / ( Im K + 1 ) ----- ( 3 )
where : Xai = 0.684 , Im = 6.97, and K = 0.311 ( input values into equation 3 )
XA2 = 0.90