Answer:
P2>S2>Cl2 is the order of bond energy of the given molecules.
Step-by-step explanation:
The bonding in each molecule is shown below:
Thus, between each P-atom, there exists a triple bond.
Between two S-atoms there exists a double bond.
Between two chlorine atoms, there exists a single bond.
As the number of bonds increases between the given atoms, then bond energy required to break the bonds also increases.
Thus, the bond order is shown below:
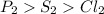 .
.